Journal updates
-
-
-
-
-
-
-
New Inspirational Note: The promise of microneedle technologies for drug delivery
The newly published inspirational note (this opens in a new tab) features innovative drug delivery technology that offers the opportunity to improve patient access and target delivery of drugs and vaccines to specific tissues.
-
-
-
-
-
-
Featured Article: June 2023
Read the featured article from the June 2023 issue! (this opens in a new tab)
Drug Delivery and Translational Research | Volume 13, issue 6 (springer.com)
-
-
-
-
2022 DDTR Best Paper of the Year
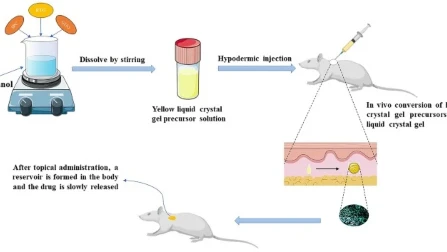
Congratulations to the authors of “A Hypotonic Gel-forming Eye Drop Provides Enhanced Intraocular Delivery of Kinase Inhibitor with Melanin-binding Properties for Sustained Protection of Retinal Ganglion Cells (this opens in a new tab)” for receiving the DDTR Best Paper of the Year Award for 2022.
-
-
-
-
Featured Articles: November 2022
Read the two equally voted for Best Papers for this issue!
Read Dr. Mandip Singh's featured article here. (this opens in a new tab)
Read Dr. Maria José Alonso's featured article here. (this opens in a new tab)
-
-
-
New Inspirational Note recently published: Chronotherapy based on modified-release hydrocortisone to restore the physiological cortisol diurnal rhythm
The newly published Inspirational Note (this opens in a new tab) features innovative drug delivery technology that replicates the physiological cortisol diurnal rhythm for chronotherapy.
-
-
Featured Article: July 2022
Read the featured article from the July 2022 issue! (this opens in a new tab)
Summary: This study introduces novel theranostic dual-targeting self-assembled peptide nanoparticles incorporated with NIRF dye and gamma-emitting radionuclide for dual-imaging functionality and enhanced therapeutic efficacy. The efficient tumor tissue targeting potential of these peptide nanoparticles was validated by the markedly enhanced therapeutic efficacy as compared to other peptide analogs reported in the literature. In vivo SPECT/NIRF images from tumor-bearing animals revealed that these nanoparticles can prominently differentiate the cancer cells from non-cancerous cells and effectively induce cell apoptosis in the targeted tumor tissues. Furthermore, in vivo therapeutic study illustrated drastic decease of tumor volume by these self-assembled peptide nanoparticles in a subcutaneous mouse model of glioblastoma.
-
Featured Article: June 2022
Read the featured article from the June 2022 issue! (this opens in a new tab)
Summary: The goal of the current research was to develop an ImM (tyrosine kinase inhibitor) containing nanoformulation and to determine if kidney delivery could be improved over traditional ImM in order to potentially treat nephritis secondary to systemic lupus erythematosus (SLEN). The glomerulus is the location within the kidney targeted in SLEN. A fish oil–based ImM oil-in-water nanoemulsion was developed and characterized for particle size, zeta potential, pH, and stability. MRL/MpJ-Faslpr (a mouse model of SLEN) and control mice (MRL-MpJ) were employed. The nanoemulsion characteristics were favorable for renal deposition (60-80 nm size, zeta potential of −6.6 to −7.8 mV, polydispersity index < 0.3, 3-day stability at 4 °C) and demonstrated limited leakage. Kidney deposition of ImM was found to be threefold higher in mice that received the nanoformulation versus naked formulation. Future strategies will define dose-efficacy and dose-toxicity relationships, and characterize and evaluate approaches to further enhance kidney deposition.
The Editorial Board of DDTR selected this article as the best paper of this issue.
-
Featured Article: May 2022
Read the featured article from the May 2022 issue! (this opens in a new tab)
Summary: This study compared drug delivery, pharmacokinetics, and treatment response after doxorubicin (DOX) conventional (c-) versus drug-eluting embolic (DEE-) transarterial chemoembolization (TACE) in a rabbit VX2 liver tumor model. Twenty-four liver tumors underwent c-TACE (n=12) or DEE-TACE (n=12). Systemic, intra-tumoral, and liver DOX levels were measured using mass spectrometry. Intra-tumoral DOX was quantified using fluorescence imaging. Percent tumor necrosis was assessed. Peak DOX concentration (µg/mL) for plasma, tumor, and liver were 0.666, 4.232, 0.270 for c-TACE, versus 0.103, 8.988, 0.610 for DEE-TACE. Area under the concentration versus time curve (µg/mL*min) for plasma, tumor tissue, and liver were 18.3, 27,078.8, 1,339.1 for c-TACE versus 16.4, 26,204.8, 1,969.6 for DEE-TACE. c-TACE achieved higher DOX coverage of tumor vs. DEE-TACE (10.8% vs. 2.3%; P=0.003). Percent tumor necrosis was similar (39% vs. 37%; P=0.806). In conclusion, in a preclinical model, both c-TACE and DEE-TACE achieved tumoricidal intra-tumoral DOX levels, though c-TACE resulted in greater tumor coverage.
The Editorial Board of DDTR selected this article as the best paper of this issue.
-
Featured Article: April 2022
Read the featured article from the April 2022 issue! (this opens in a new tab)
Summary: While eye drops are the most common ocular dosage form, eye drops for treating diseases of the posterior segment have yet to be developed. Degeneration of retinal ganglion cells (RGCs) in the retina may progress despite significant intraocular pressure lowering, suggesting that complementary neuroprotective therapies would improve glaucoma management. Here, we describe a hypotonic, thermosensitive gel-forming eye drop to effectively deliver sunitinib, with activity to enhance survival of RGCs after optic nerve injury via inhibiting dual leucine zipper kinase family. We further discovered that the combination of enhanced intraocular absorption provided by the gel-forming eye drop vehicle and the intrinsic melanin binding properties of sunitinib led to significant protection of RGCs with only once weekly eye drop dosing. For a chronic disease such as glaucoma, an effective once weekly eye drop for neuroprotection could result in greater patient adherence, and thus, greater disease management and improved patient quality of life.
The Editorial Board of DDTR selected this article as the best paper of this issue.
-
Role of drug delivery technologies in the success of COVID-19 vaccines: a perspective
This timely “Perspective” article (this opens in a new tab) discusses the roles of drug delivery technologies in developing safe and efficacious vaccines. We thank Drs. Robert Langer, Pieter Cullis, Olivia Merkel and Mark Prausnitz for sharing their perspectives and insights on this important topic.
-
Read the new Inspirational Note on vaccine delivery technology by James Goodson and Paul Rota
In this Inspirational Note (this opens in a new tab), James Goodson and Dr. Paul Rota provide insightful and timely perspective and discussion regarding the importance of vaccine delivery technology and multidisciplinary efforts to achieve global vaccination and ultimately well-being.
This article will be free to access until May 7, 2022.
-
Featured aricle: March 2022
Read the featured article from the March 2022 issue! (this opens in a new tab)
Summary: A sinonasal drug delivery system consisting of freeze-dried thermoresponsive hydrogel with degradable microspheres (MSs), called FD-TEMPS (Freeze Dried - Thermogel, Extended-release Microsphere-based delivery to the Paranasal Sinuses), was developed. Measurements of glass transition temperatures of the maximally freeze concentrated solutions informed optimization of the thermogel. Using a higher molecular weight polyethylene glycol (2000 Da) with poly(N-isopropylacrylamide) resulted in freeze-dried gel with a brittle texture, porous structure, and low residual moisture (<3%). When combined with poly(lactic-co-glycolic acid) MSs and freeze dried, FD-TEMPS exhibited enhanced shelf-stability with maintained MS morphology and initial release kinetics following 6-weeks storage under ambient conditions. Furthermore, FD-TEMPS remained in place after application to a simulated mucosal surface, suggesting that it could be more uniformly distributed along the sinonasal mucosa. Freeze drying enables this sinonasal delivery system to be stored as a ready-to-use product for better ease of clinical translation without compromising thermoresponsive or sustained release characteristics.
The Editorial Board of DDTR selected this article as the best paper of this issue.
-
-
-
Featured Article: October 2021
Read the featured article from the October 2021 issue! (this opens in a new tab)
Summary: In current investigation, combinatorial delivery of anti-neoplastic drug and siRNA was achieved to enhance the effectiveness of cisplatin in A549 lung cancer cell line. The formulated co-loaded nanocarriers imparted stability to the siRNA in presence of serum and delivered the payload efficiently inside the cells as evident from the transfection efficiency study besides showing improvement in loading efficiency of the drug as well as cytotoxicity as compared to the free drug. Delivery of ABCC3 siRNA complexed HNCs for ABCC3 protein exerted knock-down of expression leading to sensitization of cancer cells to cisplatin. A significant reduction in tumor growth along with superior pharmacokinetics profile was observed with the formulation. Thus, this approach has potential for effective management of cancer using genomic approach with improved therapeutic index and could be able to reduce dose dependent toxicity of the cisplatin.
The Editorial Board of DDTR selected this article as the best paper of this issue. This article will be free to access until November 24, 2021.
-
2020 DDTR Best Paper of the Year
Congratulations to the authors of Translational studies of intravenous and intracerebroventricular routes of administration for CNS cellular biodistribution for BMN 250, an enzyme replacement therapy for the treatment of Sanfilippo type B (this opens in a new tab) for receiving the DDTR Best Paper of the Year Award for 2021 at the 2021 CRS virtual meeting.
Summary: Treatment of the neurological manifestations of lysosomal storage diseases remains an unmet medical need. BMN 250 is being developed as an enzyme replacement therapy administered directly into the brain ventricles via the intracerebroventricular (ICV) route for treating Sanfilippo type B. Patients with this rare neurological disease have deficient lysosomal alpha-N-acetylglucosaminidase (NAGLU) enzyme activity. Unlike the ICV route that readily provides biodistribution of therapy to target cells, we show in wild-type and disease animal models that intravenously-administered enzyme does not reach the target neuronal cells. The limited pharmacological response following intravenous administration is likely attributed to the clearance of substrate in endothelial cells that restrict biodistribution to target cells in the brain. Representative staining from superficial medulla oblongata from cynomolgus monkey received BMN 250 via the IV route shows that all NAGLU staining is co-localized with CD31-positive endothelial cells, indicating that IV administered NAGLU does not reach the target neurons. In contrast, NAGLU co-localizes with the neurons following ICV administration.
This article was published Open Access and is freely accessible to anyone.
-
A message from the incoming President of the Controlled Release Society
(this opens in a new tab)Incoming CRS President Ben Boyd provides his thoughts on the 2020 Impact Factor, the journal itself and the Best Paper of the Year awarded at the 2021 CRS Virtual Annual Meeting.
Click here (this opens in a new tab) to see the video
-
Featured article: August 2021
Read the featured article for the August 2021 issue:
Summary: The use of natural polysaccharides in nanovaccine carriers impacts immunogenicity and antigen delivery efficacy. In this work, novel carboxymethyl β-glucan:chitosan nanoparticles were developed to deliver ovalbumin (OVA) to immune cells in lymph nodes. Fluorescently labelled OVA-loaded nanoparticles accumulated in the medullary and interfollicular areas of the popliteal lymph node, 12 hours post-subcutaneous injection into the footpad of C57/BL6 mice. The ability of β-glucan to interact with the dendritic cell receptor Dectin-1 is likely responsible for efficient immune cell targeting observed with our formulation. Thermostability was also achieved through lyophilization of antigen-loaded carriers, which remained stable in a powder form for up to one month at 40 °C. Moreover, subcutaneous immunization of mice with a single dose of the OVA-loaded nanoparticles led to induced T cell proliferation and antibody responses, comparable to those achieved with alum-adsorbed OVA, illustrating the potential of these novel nanocarriers in vaccination.
This paper was selected as the best paper in the August 2021 issue by the editors and guest editors.
-
Inspirational Note on oral peptide delivery
In this Inspirational Note (this opens in a new tab), Dr. Andrew Lewis and coauthors feature an amazing and innovative discovery that is changing the paradigm of oral peptide delivery.
This article will be free to access until July 25, 2021.
-
Inspirational Note by Lara Milane and Mansoor Amiji
In this Inspirational Note (this opens in a new tab), Drs. Lara Milane and Mansoor Amiji share their insights and reflections on the impact of the recently approved SARS-CoV-2 mRNA vaccines on the present and future of translational nanomedicines.
-
2020 DDTR Best Paper of the Year
Congratulations to the authors of Translational studies of intravenous and intracerebroventricular routes of administration for CNS cellular biodistribution for BMN 250, an enzyme replacement therapy for the treatment of Sanfilippo type B (this opens in a new tab) for winning the DDTR Best Paper of the Year Award for 2020.
Summary: Treatment of the neurological manifestations of lysosomal storage diseases remains an unmet medical need. BMN 250 is being developed as an enzyme replacement therapy administered directly into the brain ventricles via the intracerebroventricular (ICV) route for treating Sanfilippo type B. Patients with this rare neurological disease have deficient lysosomal alpha-N-acetylglucosaminidase (NAGLU) enzyme activity. Unlike the ICV route that readily provides biodistribution of therapy to target cells, we show in wild-type and disease animal models that intravenously-administered enzyme does not reach the target neuronal cells. The limited pharmacological response following intravenous administration is likely attributed to the clearance of substrate in endothelial cells that restrict biodistribution to target cells in the brain. Representative staining from superficial medulla oblongata from cynomolgus monkey received BMN 250 via the IV route shows that all NAGLU staining is co-localized with CD31-positive endothelial cells, indicating that IV administered NAGLU does not reach the target neurons. In contrast, NAGLU co-localizes with the neurons following ICV administration.
-
Inspirational Note by Alexander Florence
In this Inspirational Note (this opens in a new tab), Dr. Alexander Florence shares his reflections on the development of nanosystems designed for the delivery of biologically active agents.
This is an Open Access article and will be free to read in perpetuity.
-
New Reviewer Recruitment
Drug Delivery and Translational Research invites you to join our team of reviewers. To be considered, please send your CV to the Editorial Office (this opens in a new tab). -
Featured article: February 2021
Read the featured article for the February 2021 issue:
Summary: Intra-articular hyaluronic acid (HA) injection is an effective and safe treatment for patients with mild-to-moderate knee osteoarthritis, which relieves pain and improves joint function. Injection through medial midpatellar (MMP) route is observed to provide a greater coverage of HA throughout the vulnerable regions of the articular cartilage in osteoarthritic knee compare to anteromedial (AM) route. Specifically, intra-articular HA with MMP injection route showed a nearly full coverage of patellofemoral joint and a large coverage of medial femorotibial joint, as well as a half coverage of lateral femorotibial joint. Correspondingly, injection through the MMP route is superior to AM route in achieving pain relief and global patient satisfaction. To this end, intra-articular HA injection through the MMP route is recommended for patients with mild-to-moderate knee osteoarthritis in the future clinical practice.
This paper was selected as the best paper in the February 2021 issue by the editors.
This article will be Free-to-Access until March 20, 2021.
-
Inspirational Note by Dr. Marianne Ashford
(this opens in a new tab)In this Inspirational Note (this opens in a new tab), Dr. Marianne Ashford outlines how the field of drug delivery has evolved over the past decades and shares skills, capabilities and behaviors instrumental and critical for the success of next-generation medicines.
This article will be free to access until January 17, 2021.
-
Featured articles, December 2020 issue
Read the best article from the December 2020 issue:
Summary: onvention-enhanced delivery (CED) is developed to infuse drugs directly into the brain using a catheter with a continuous positive pressure, thus overcoming the limitations of intravascular delivery such as blood-brain barrier. However, tissue distribution and retention of the infused drugs in the brain are significantly hindered by microenvironmental factors of the brain such as the extracellular matrix and lymphatic drainage system. In this work, we precisely engineered liposomal formulations to improve the therapeutic efficacy of CED for the treatment of glioblastoma. We found that PEGylated liposomal formulations incorporated with cationic lipids at the proper molar ratio exhibited specific cellular uptake in the glioblastoma cells in vitro, and efficient tissue distribution and retention in the brain tumor after CED. We believe that liposomal drugs engineered based on the biophysical properties of disease microenvironments will be widely used to improve the therapeutic efficacy of CED for the treatment of brain diseases.
This paper was selected by the Guest Editors and Main Editors as the best paper from the 22nd International Symposium on Microencapsulation.This article will be Free-to-Access until 2 January, 2021.
-
A Blast from the Past – the Top Ten Most Cited Articles Published in 2018-2019
Here are the ten most cited papers published from 2018-2019 in Drug Delivery and Translational Research. Together they represent high-quality research published in DDTR on a wide range of topics as judged and cited by your peers. Discover them now!
-
Featured article for October 2020 issue
Read the featured article from the October 2020 issue! (this opens in a new tab)
Summary: The effect of local anesthetics, particularly those which are hydrophilic, such as tetrodotoxin (TTX), is impeded by tissue barriers that restrict access to individual nerve cells. Insonation has been used to disrupt vascular and cell membranes, which can facilitate transport of substances across biological barriers. Using an in vivo model of local anesthetic delivery, we investigated the effect of acoustic intensity on insonation-mediated delivery of local anesthetics to the peripheral nerve. We found that insonation at acoustic intensity greater than 0.5 W/cm2 was capable of enhancing TTX delivery to the peripheral nerve but not bupivacaine, a more hydrophobic local anesthetic. The finding here suggests that the effect of insonation on local drug delivery to the peripheral nerve depends on the hydrophilic/hydrophobic balance of the drug.
-
Featured Article: August 2020
Read the article! (this opens in a new tab)
Summary: Sorafenib, a first-line drug for advanced liver cancer, exhibits low and inconsistent oral bioavailability due primarily to its poor aqueous solubility, extensive first-pass metabolism and high level drug efflux by P-gp. Administration of sorafenib at high doses, necessary for meaningful therapeutic action, increases the intensity of associated side effects. Therefore, efforts were undertaken to develop supersaturated Type-III self-emulsifying delivery system for efficient and safe delivery of sorafenib, capitalizing the ease of preparation, biocompatibility, biodegradability, ability to bypass first-pass metabolism, high drug loading capacity, colloidal stability for long-term storage, and excellent scalability. Promising outcomes in diverse experimental studies corroborate immense potential of the system in augmenting the chemotherapeutic efficacy of sorafenib while minimizing safety concern.
This paper was selected as the best paper of the August 2020 issue by the Guest Editors and Main Editors. This article will be Free-to-Access until 24 September, 2020.
-
Best article from the June 2020 special issue on Nanomedicines
Read the best article from the June 2020 issue! (this opens in a new tab)
Summary: Bevacizumab is a monoclonal antibody that specifically inactivates VEGF-A, resulting in a decrease of the development of tumor blood vessels and its growth. Currently, it is employed in the treatment of different types of tumors, particularly colorectal cancer. As for other biologicals, the benefits of bevacizumab are limited by a low capability to penetrate into the tumors that, in addition, may increase the risk of acquiring resistance. In this work, the effect of the nanoencapsulation of bevacizumab (in human serum albumin nanoparticles) was evaluated on a xenograft model of human colorectal cancer.
The resulting nanoparticles demonstrated to be more effective in decreasing the glycolysis and metabolic tumor volume than the conventional treatment. This improvement was associated with a significantly higher accumulation of the monoclonal antibody in the tumor tissues when nanoencapsulated in human serum albumin nanoparticles.
This article was selected as the best article of the Nanomedicines Special Issue by the CRS Nanomedicine and Nanoscale Delivery Focus Group.